Background:
Capacitive-resistive electric transfer (CRET) is a non-invasive therapeutic strategy that applies radiofrequency electric currents within the 400–600 kHz range to tissue repair and regeneration. Previous studies by our group have shown that 48 h of intermittent exposure to a 570 kHz CRET signal at a subthermal density of 50 μA/mm2 causes significant changes in the expression and activation of cell cycle control proteins, leading to cycle arrest in human cancer cell cultures. The present study investigates the relevance of the signal frequency in the response of the human neuroblastoma cell line NB69 to subthermal electric treatment with four different signal frequency currents within the 350–650 kHz range.
Conclusions:
The understanding of the mechanisms underlying the ability of slowing down cancer cell growth through electrically-induced changes in the expression of proteins involved in the control of cell proliferation and apoptosis might afford new insights in the field of oncology.
Link to Article
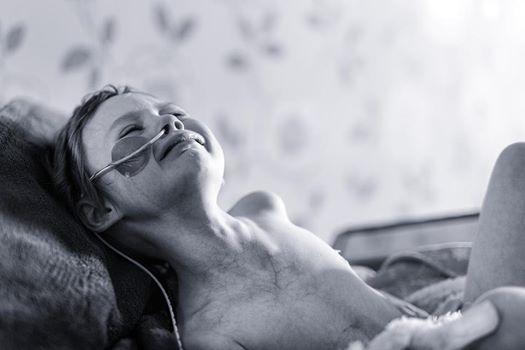
An attempt to wake up the medical community to accept research done in the last 100 years proving that electromagnetic energy can replace brutal chemotherapy. Photo taken by a professional photographer, of his own daughter being treated for Neuroblastoma. The power of the image encouraged Andy to share it with others in order to highlight the 'real' face of childhood cancer. She died. The average cost for such treatment is in the order of 500k+.
Saturday, September 28, 2019
Friday, September 27, 2019
The variable electric field of the membrane /Questioning the HodgkinHuxley model
Hodgkin and Huxley built their theoretical model of initiation and propagation of action potential on the work of their predecessors but also that of their contemporaries.
Bernstein was the initiator of membrane theory but Hodgkin, Katz and especially Goldman sought to improve it.
The integration of the constant electric field should have allowed a better knowledge and computation of the membrane potential.
The study of the variations in the electric field that would exist through the membrane brings harsh contradictions in the hypotheses of the HH model.
Link to Article
Bernstein was the initiator of membrane theory but Hodgkin, Katz and especially Goldman sought to improve it.
The integration of the constant electric field should have allowed a better knowledge and computation of the membrane potential.
The study of the variations in the electric field that would exist through the membrane brings harsh contradictions in the hypotheses of the HH model.
Link to Article
Monday, September 16, 2019
The combined treatment of 150 kHz tumor treating fields (TTFields) and cisplatin or pemetrexed inhibits mesothelioma cells in vitro and in vivo.
Background: Malignant pleural mesothelioma (MPM) is an aggressive thoracic cancer mostly linked to asbestos exposure. The standard of care (SOC) therapy for unresectable MPM is cisplatin plus pemetrexed. Treating Fields (TTFields) therapy is an effective anti-neoplastic treatment modality delivered via noninvasive application of low intensity, intermediate frequency, alternating electric fields. We explored the potential use of TTFields alone and in combination with SOC as a treatment for MPM. Methods: NCI-H2052 and MSTO-211H cells were treated at various TTFields frequencies for 72 hours using the inovitro system. The combined treatment of TTFields and cisplatin or pemetrexed was tested by applying TTFields at the optimal frequency together with various drug concentrations. Cell counts, clonogenic potential and induction of apoptosis were determined. TTFields (1.2 V/cm) were applied for 8 days to rats injected to the intrapleural cavity with IL-45 cells, and overall survival was tested. Results: TTFields optimal frequency was 150 kHz for both human cell lines. TTFields application (1.1 V/cm, 72 hours) at 150 kHz led to 45%-51% reduction in cell counts and 46-64%% additional reduction in clonogenic potential. The combined treatment of TTFields and cisplatin or pemetrexed led to a significant reduction in cell count, induction of apoptosis and reduced clonogenic potential as compared to each modality alone (p < 0.0001(. TTFields significantly prolonged the survival of rats compared to control group. Safety studies did not reveal any adverse events associated with 150 kHz TTFields application to the rat torso. Conclusions: These results demonstrate that TTFields can be an effective treatment against mesothelioma and the combination with cisplatin or pemetrexed may further enhance treatment efficacy. These results are in consistency with the recent phase 2 study (EF-23 trial) that showed improved overall survival for combined treatment as compared to historical control with no increase in systemic toxicity.
Article
Article
Friday, September 13, 2019
Electromagnetic fields alter the motility of metastatic breast cancer cells
Interactions between cells and their environment influence key physiologic processes such as their propensity to migrate.
However, directed migration controlled by extrinsically applied electrical signals is poorly understood.
Using a novel microfluidic platform, we found that metastatic breast cancer cells sense and respond to the net direction of weak (∼100 µV cm−1), asymmetric, non-contact induced Electric Fields (iEFs).
iEFs inhibited EGFR (Epidermal Growth Factor Receptor) activation, prevented formation of actin-rich filopodia, and hindered the motility of EGF-treated breast cancer cells.
The directional effects of iEFs were nullified by inhibition of Akt phosphorylation.
Moreover, iEFs in combination with Akt inhibitor reduced EGF-promoted motility below the level of untreated controls.
These results represent a step towards isolating the coupling mechanism between cell motility and iEFs, provide valuable insights into how iEFs target multiple diverging cancer cell signaling mechanisms, and demonstrate that electrical signals are a fundamental regulator of cancer cell migration.
Link to article
However, directed migration controlled by extrinsically applied electrical signals is poorly understood.
Using a novel microfluidic platform, we found that metastatic breast cancer cells sense and respond to the net direction of weak (∼100 µV cm−1), asymmetric, non-contact induced Electric Fields (iEFs).
iEFs inhibited EGFR (Epidermal Growth Factor Receptor) activation, prevented formation of actin-rich filopodia, and hindered the motility of EGF-treated breast cancer cells.
The directional effects of iEFs were nullified by inhibition of Akt phosphorylation.
Moreover, iEFs in combination with Akt inhibitor reduced EGF-promoted motility below the level of untreated controls.
These results represent a step towards isolating the coupling mechanism between cell motility and iEFs, provide valuable insights into how iEFs target multiple diverging cancer cell signaling mechanisms, and demonstrate that electrical signals are a fundamental regulator of cancer cell migration.
Link to article
Capacitive-resistive electric transfer (CRET) is a non-invasive therapeutic strategy
Background: Capacitive-resistive electric transfer (CRET) is a non-invasive therapeutic strategy that applies radiofrequency electric currents within the 400-600 kHz range to tissue repair and regeneration.
Previous studies by our group have shown that 48 h of intermittent exposure to a 570 kHz CRET signal at a subthermal density of 50 μA/mm2 causes significant changes in the expression and activation of cell cycle control proteins, leading to cycle arrest in human cancer cell cultures.
The present study investigates the relevance of the signal frequency in the response of the human neuroblastoma cell line NB69 to subthermal electric treatment with four different signal frequency currents within the 350-650 kHz range.
Methods: Trypan blue assay, flow cytometry, immunofluorescence and immunoblot were used to study the effects of subthermal CRET currents on cell viability, cell cycle progression and the expression of several marker proteins involved in NB69 cell death and proliferation.
Results: The results reveal that among the frequencies tested, only a 448 kHz signal elicited both proapoptotic and antiproliferative, statistically significant responses.
The apoptotic effect would be due, at least in part, to significant changes induced by the 448 kHz signal in the expression of p53, Bax and caspase-3.
The cytostatic response was preceded by alterations in the kinetics of the cell cycle and in the expression of proteins p-ERK1/2, cyclin D1 and p27, which is consistent with a potential involvement of the EGF receptor in electrically induced changes in the ERK1/2 pathway.
This receives additional support from results indicating that the proapototic and antiproliferative responses to CRET can be transiently blocked when the electric stimulus is applied in the presence of PD98059, a chemical inhibitor of the ERK1/2 pathway.
Conclusion: The understanding of the mechanisms underlying the ability of slowing down cancer cell growth through electrically-induced changes in the expression of proteins involved in the control of cell proliferation and apoptosis might afford new insights in the field of oncology.
Article Link
Previous studies by our group have shown that 48 h of intermittent exposure to a 570 kHz CRET signal at a subthermal density of 50 μA/mm2 causes significant changes in the expression and activation of cell cycle control proteins, leading to cycle arrest in human cancer cell cultures.
The present study investigates the relevance of the signal frequency in the response of the human neuroblastoma cell line NB69 to subthermal electric treatment with four different signal frequency currents within the 350-650 kHz range.
Methods: Trypan blue assay, flow cytometry, immunofluorescence and immunoblot were used to study the effects of subthermal CRET currents on cell viability, cell cycle progression and the expression of several marker proteins involved in NB69 cell death and proliferation.
Results: The results reveal that among the frequencies tested, only a 448 kHz signal elicited both proapoptotic and antiproliferative, statistically significant responses.
The apoptotic effect would be due, at least in part, to significant changes induced by the 448 kHz signal in the expression of p53, Bax and caspase-3.
The cytostatic response was preceded by alterations in the kinetics of the cell cycle and in the expression of proteins p-ERK1/2, cyclin D1 and p27, which is consistent with a potential involvement of the EGF receptor in electrically induced changes in the ERK1/2 pathway.
This receives additional support from results indicating that the proapototic and antiproliferative responses to CRET can be transiently blocked when the electric stimulus is applied in the presence of PD98059, a chemical inhibitor of the ERK1/2 pathway.
Conclusion: The understanding of the mechanisms underlying the ability of slowing down cancer cell growth through electrically-induced changes in the expression of proteins involved in the control of cell proliferation and apoptosis might afford new insights in the field of oncology.
Article Link
Tuesday, September 10, 2019
Crucial functions associated with phototropism and circadian clocks
"Cryptochromes are flavoproteins whose photochemistry is important for crucial functions associated with phototropism and circadian clocks. In this report, we, for the first time, observed a magnetic response of the cryptochrome 1 (CRY1) immobilized at a gold electrode with illumination of blue light. These results present the magnetic field-enhanced photoinduced electron transfer of CRY1 to the electrode by voltammetry, exhibiting magnetic responsive rate constant and electrical current changes. A mechanism of the electron transfer, which involves photoinduced radicals in the CRY, is sensitive to the weak magnetic field; and the long-lived free radical FAD•– is responsible for the detected electrochemical Faradaic current. As a photoreceptor, the finding of a 5.7% rate constant change in electron transfer corresponding to a 50 μT magnetic field may be meaningful in regulation of magnetic field signaling and circadian clock function under an electromagnetic field."
https://www.researchgate.net/publication/324885021_Magnetoreception_of_Photoactivated_Cryptochrome_1_in_Electrochemistry_and_Electron_Transfer
https://www.researchgate.net/publication/324885021_Magnetoreception_of_Photoactivated_Cryptochrome_1_in_Electrochemistry_and_Electron_Transfer
Monday, September 2, 2019
Changes in chromatin accessibility and consequently in the expression of anti-inflammatory mediators and in cell differentiation.
In this study, we investigated the effects of specific low frequency electromagnetic fields sequences on U937 cells, an in vitro model of human monocyte/macrophage differentiation. U937 cells were exposed to electromagnetic stimulation by means of the SyntheXer system using two similar sequences, XR-BC31 and XR-BC31/F. Each sequence was a time series of twenty-nine wave segments. Here, we report that exposure (4 days, once a day) of U937 cells to the XR-BC31 setting, but not to the XR-BC31/F, resulted in increased expression of the histone demethylase KDM6B along with a global reduction in histone H3 lysine 27 (H3K27) tri-methylation. Furthermore, exposure to the XR-BC31 sequence induced differentiation of U937 cells towards a macrophage-like phenotype displaying a KDM6B dependent increase in expression and secretion of the anti-inflammatory interleukins (ILs), IL-10 and IL-4. Importantly, all the observed changes were highly dependent on the sequence's nature. Our results open a new way of interpretation for the effects of low frequency electromagnetic fields observed in vivo. Indeed, it is conceivable that a specific low frequency electromagnetic fields treatment may cause changes in chromatin accessibility and consequently in the expression of anti-inflammatory mediators and in cell differentiation.
https://www.researchgate.net/publication/328304138_Specific_low_frequency_electromagnetic_fields_induce_epigenetic_and_functional_changes_in_U937_cells
https://www.researchgate.net/publication/328304138_Specific_low_frequency_electromagnetic_fields_induce_epigenetic_and_functional_changes_in_U937_cells
Subscribe to:
Posts (Atom)