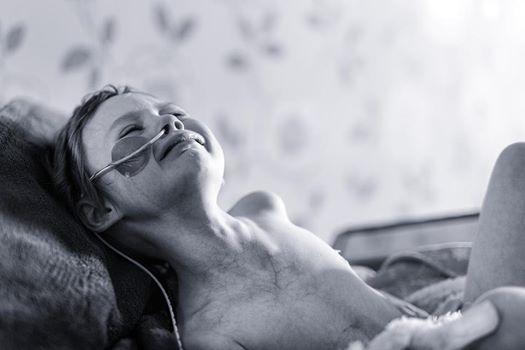
An attempt to wake up the medical community to accept research done in the last 100 years proving that electromagnetic energy can replace brutal chemotherapy. Photo taken by a professional photographer, of his own daughter being treated for Neuroblastoma. The power of the image encouraged Andy to share it with others in order to highlight the 'real' face of childhood cancer. She died. The average cost for such treatment is in the order of 500k+.
Monday, January 30, 2017
Changes in cell death of peripheral blood lymphocytes isolated from children with acute lymphoblastic leukemia upon stimulation with 7 Hz, 30 mT pulsed electromagnetic field.
Tuesday, January 24, 2017
Bioelectromagnetic Healing, its History and a Rationale for its Use
This paper gives an excellent historical account of Pulsed Electromagnetic Field therapy for cancer (and why it was rejected by the established medical sector), and technical aspects of it:
Abstract
Bioelectromagnetics (BEMs) is the study of the effect of electromagnetic fields on biological systems.1 Though electromagnetic fields have sometimes been associated with potential for harm to the body, there are many BEM instruments and devices re-emerging in the 21st century, based on high voltage Tesla coils, that apparently bring beneficial health improvements to human organisms. The Tesla coil class of therapy devices constitute pulsed electromagnetic fields (PEMF) that deliver broadband, wide spectrum, nonthermal photons and electrons deep into biological tissue. Electromedicine or electromagnetic healing are the terms applied to such developments in the ELF, RF, IR, visible or UV band. With short term, non-contacting exposures of several minutes at a time, such high voltage Tesla PEMF devices may represent the ideal, noninvasive therapy of the future, accompanied by a surprising lack of harmful side effects. A biophysical rationale for the benefits of BEM healing a wide variety of illnesses including cancer, proposes a correlation between a bioelectromagnetically restored transmembrane potential, and the electron transport across cell membranes by electroporation, with normal cell metabolism and immune system enhancement. The century-long historical record of these devices is also traced, revealing questionable behavior from the medical and public health institutions toward such remarkable innovations. This report also reviews the highlights of several BEM inventions but does not attempt to present an exhaustive nor comprehensive review of bioelectromagnetic healing devices. History of Bioelectromagnetic Healing Historically, as far back as 1890, the American Electro-Therapeutic Association conducted annual conferences on the therapeutic use of electricity and electrical devices by physicians on ailing patients. Some involved current flow through the patient, while others were electrically powered devices. At first, only direct current (DC) devices were utilized in the medical doctor's office for relieving pain and vibrating female patients who were routinely diagnosed with "hysteria."
The Anti-Tumor Effect of A3 Adenosine Receptors Is Potentiated by Pulsed Electromagnetic Fields in Cultured Neural Cancer Cells
Abstract
A(3) adenosine receptors (ARs) play a pivotal role in the development of cancer and their activation is involved in the inhibition of tumor growth. The effects of pulsed electromagnetic fields (PEMFs) on cancer have been controversially discussed and the detailed mechanisms are not yet fully understood. In the past we have demonstrated that PEMFs increased A(2A) and A(3)AR density and functionality in human neutrophils, human and bovine synoviocytes, and bovine chondrocytes. In the same cells, PEMF exposure increased the anti-inflammatory effect mediated by A(2A) and/or A(3)ARs. The primary aim of the present study was to evaluate if PEMF exposure potentiated the anti-tumor effect of A(3)ARs in PC12 rat adrenal pheochromocytoma and U87MG human glioblastoma cell lines in comparison with rat cortical neurons. Saturation binding assays and mRNA analysis revealed that PEMF exposure up-regulated A(2A) and A(3)ARs that are well coupled to adenylate cyclase activity and cAMP production. The activation of A(2A) and A(3)ARs resulted in the decrease of nuclear factor-kappa B (NF-kB) levels in tumor cells, whilst only A(3)ARs are involved in the increase of p53 expression. A(3)AR stimulation mediated an inhibition of tumor cell proliferation evaluated by thymidine incorporation. An increase of cytotoxicity by lactate dehydrogenase (LDH) release and apoptosis by caspase-3 activation in PC12 and U87MG cells, but not in cortical neurons, was observed following A(3)AR activation. The effect of the A(3)AR agonist in tumor cells was enhanced in the presence of PEMFs and blocked by using a well-known selective antagonist. Together these results demonstrated that PEMF exposure significantly increases the anti-tumor effect modulated by A(3)ARs.
Effect of Pulsed Electromagnetic Field on MMP-9 and TIMP-1 Levels in Chondrosarcoma Cells Stimulated with IL-1β
Abstract
Chondrosarcoma, the second most common type of bone malignancy, is characterized by distant metastasis and local invasion. Previous studies have shown that treatment by pulsed electromagnetic field (PEMF) has beneficial effects on various cancer cells. In this study, we investigated the effects of PEMF applied for 3 and 7 days on the matrix metalloproteinase (MMP) levels in chondrosarcoma SW1353 cells stimulated with two different doses of IL-1β. SW1353 cells were treated with (0.5 and 5 ng/ml) IL-1β and PEMF exposure was applied either 3 or 7 days. MMP-9 and TIMP-1 levels were measured in conditioned media by enzyme-linked immunosorbent assay. The results were relative to protein levels. Statistical analyses were performed using one-way analysis of variance (ANOVA). P<0.05 was considered significant. PEMF treatment significantly decreased MMP-9 protein levels in human chondrosarcoma cells stimulated with 0.5 ng/ml IL-1β at day 7, whereas it did not show any effect on cells stimulated with 5 ng/ml IL-1β. There was no significant change in TIMP-1 protein levels either by IL-1β stimulation or by PEMF treatment. The results of this study showed that PEMF treatment suppressed IL-1β-mediated upregulation of MMP-9 protein levels in a dual effect manner. This finding may offer new perspectives in the therapy of bone cancer.
Electromagnetic effects-From cell biology to medicine
Abstract
In this review we compile and discuss the published plethora of cell biological effects which are ascribed to electric fields (EF), magnetic fields (MF) and electromagnetic fields (EMF). In recent years, a change in paradigm took place concerning the endogenously produced static EF of cells and tissues. Here, modern molecular biology could link the action of ion transporters and ion channels to the "electric" action of cells and tissues. Also, sensing of these mainly EF could be demonstrated in studies of cell migration and wound healing. The triggers exerted by ion concentrations and concomitant electric field gradients have been traced along signaling cascades till gene expression changes in the nucleus. Far more enigmatic is the way of action of static MF which come in most cases from outside (e.g. earth magnetic field). All systems in an organism from the molecular to the organ level are more or less in motion. Thus, in living tissue we mostly find alternating fields as well as combination of EF and MF normally in the range of extremely low-frequency EMF. Because a bewildering array of model systems and clinical devices exits in the EMF field we concentrate on cell biological findings and look for basic principles in the EF, MF and EMF action. As an outlook for future research topics, this review tries to link areas of EF, MF and EMF research to thermodynamics and quantum physics, approaches that will produce novel insights into cell biology.
Monday, January 2, 2017
Modification of p21 level and cell cycle distribution by 50 Hz magnetic fields in human SH-SY5Y neuroblastoma cells
Abstract
Purpose: In our previous studies, exposure to extremely low frequency (ELF) magnetic fields (MFs) altered responses to DNA damage caused by menadione. The aim of this study was to evaluate possible ELF MF induced changes in proteins involved in DNA damage responses and in cell cycle distribution.
Materials and methods: Based on our previous studies, the exposure protocol included pre-exposure of human SH-SY5Y neuroblastoma cells to a 50 Hz, 100 µT MF for 24 h prior to a 3-h menadione treatment. As DNA damage responses are relatively fast processes, a 1-h menadione treatment was also included in the experiments. The menadione concentrations used were 1, 10, 15, 20, and 25 µM. Immunoblotting was used to assess the levels of DNA damage response-related proteins (γ-H2AX, Chk1, phospho-Chk1, p21, p27, and p53), while the level of DNA damage was assessed by the alkaline Comet assay. Cell cycle distribution was assayed by SYTOX Green staining followed by flow cytometry analysis.
Results: The main findings in MF-exposed cells were decreased p21 protein level after the 1-h menadione treatment, as well as increased proportion of cells in the G1 phase and decreased proportion of S phase cells after the 3-h menadione treatment. These effects were detectable also in the absence of menadione.
Conclusions: The results indicate that MF exposure can alter the G1 checkpoint response and that the p21 protein may be involved in early responses to MF exposure.
Materials and methods: Based on our previous studies, the exposure protocol included pre-exposure of human SH-SY5Y neuroblastoma cells to a 50 Hz, 100 µT MF for 24 h prior to a 3-h menadione treatment. As DNA damage responses are relatively fast processes, a 1-h menadione treatment was also included in the experiments. The menadione concentrations used were 1, 10, 15, 20, and 25 µM. Immunoblotting was used to assess the levels of DNA damage response-related proteins (γ-H2AX, Chk1, phospho-Chk1, p21, p27, and p53), while the level of DNA damage was assessed by the alkaline Comet assay. Cell cycle distribution was assayed by SYTOX Green staining followed by flow cytometry analysis.
Results: The main findings in MF-exposed cells were decreased p21 protein level after the 1-h menadione treatment, as well as increased proportion of cells in the G1 phase and decreased proportion of S phase cells after the 3-h menadione treatment. These effects were detectable also in the absence of menadione.
Conclusions: The results indicate that MF exposure can alter the G1 checkpoint response and that the p21 protein may be involved in early responses to MF exposure.
Subscribe to:
Posts (Atom)