Although theories regarding the role of sequence-specific DNA resonance in biology have abounded for over 40 years, the published evidence for it is lacking. Here, the authors reasoned that for sustained resonance signaling, the number of oscillating DNA sequences per genome should be exceptionally high and that, therefore, genomic repeats of various sizes are good candidates for serving as resonators. Moreover, it was suggested that for the two DNA sequences to resonate, they do not necessarily have to be identical. Therefore, the existence of sequences differing in the primary sequence but having similar resonating sub-structures was proposed. It was hypothesized that such sequences, named HIDERs, would be enriched in the genomes of multicellular species. Specifically, it was hypothesized that delocalized electron clouds of purine-pyrimidine sequences could serve as the basis of HIDERs. The consequent genomic analysis confirmed the enrichment of purine-pyrimidine HIDERs in a few selected genomes of mammals, an insect, and a plant, compared to randomized sequence controls. Similarly, it was suggested that hypothetical delocalized proton clouds of the hydrogen bonds of multiple stacked bases could serve as sequence-dependent hydrogen-bond-based HIDERs. Similarly, the enrichment of such HIDERs was observed. It is suggested that these enrichments are the first evidence in support of sequence-specific resonance signaling in the genome.
Link to Article
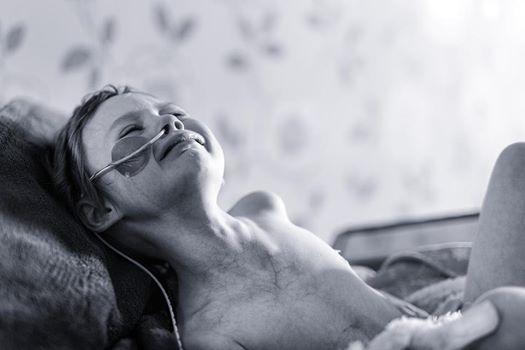
An attempt to wake up the medical community to accept research done in the last 100 years proving that electromagnetic energy can replace brutal chemotherapy. Photo taken by a professional photographer, of his own daughter being treated for Neuroblastoma. The power of the image encouraged Andy to share it with others in order to highlight the 'real' face of childhood cancer. She died. The average cost for such treatment is in the order of 500k+.
Saturday, November 30, 2019
Tuesday, November 26, 2019
KCa and Ca2 + Channels: The complex thought
Potassium channels belong to the largest and the most diverse super-families of ion channels. Among them, Ca2 +-activated K+ channels (KCa) comprise many members. Based on their single channel conductance they are divided into three subfamilies: big conductance (BKCa), intermediate conductance (IKCa) and small conductance (SKCa; SK1, SK2 and SK3). Ca2 + channels are divided into two main families, voltage gated/voltage dependent Ca2 + channels and non-voltage gated/voltage independent Ca2 + channels. Based on their electrophysiological and pharmacological properties and on the tissue where there are expressed, voltage gated Ca2 + channels (Cav) are divided into 5 families: T-type, L-type, N-type, P/Q-type and R-type Ca2 +. Non-voltage gated Ca2 + channels comprise the TRP (TRPC, TRPV, TRPM, TRPA, TRPP, TRPML and TRPN) and Orai (Orai1 to Orai 3) families and their partners STIM (STIM1 to STIM2). A depolarization is needed to activate voltage-gated Ca2 + channels while non-voltage gated Ca2 + channels are activated by Ca2 + depletion of the endoplasmic reticulum stores (SOCs) or by receptors (ROCs). These two Ca2 + channel families also control constitutive Ca2 + entries. For reducing the energy consumption and for the fine regulation of Ca2 +, KCa and Ca2 + channels appear associated as complexes in excitable and non-excitable cells. Interestingly, there is now evidence that KCa - Ca2 + channel complexes are also found in cancer cells and contribute to cancer-associated functions such as cell proliferation, cell migration and the capacity to develop metastases. This article is part of a Special Issue entitled: Calcium Signaling In Health and Disease.
Article Link
Article Link
Friday, November 1, 2019
Voltage Gated Sodium Channels in Cancer and Their Potential Mechanisms of Action
Voltage gated sodium channels (VGSC) are implicated in cancer cell invasion and metastasis. However, the mechanism by which VGSC increase cell invasiveness and probability of metastasis is still unknown. In this review we outline lesser known functions of VGSC outside of action potential propagation, and the current understanding of the effects of VGSC in cancer. Finally, we discuss possible downstream effects of VGSC activation in cancer cells. After extensive review of the literature, the most likely role of VGSC in cancer is in the invadopodia, the leading edge of metastatic cancer cells. Sodium gradients are used to drive many biological processes in the body, and invadopodia may be similar. The function of the sodium hydrogen exchanger (NHE) and sodium calcium exchanger (NCX) are driven by sodium gradients. Voltage gated calcium channels, activated by membrane depolarization, are also capable of becoming activated in response to VGSC activity. Changes to hydrogen ion exchange or calcium handling have functional consequences for invadopodia and would explain the relationship between VGSC expression and invasiveness of cancer cells.
Link to Article
Link to Article
Subscribe to:
Posts (Atom)