Exposure to man-made electromagnetic fields (EMFs), which increasingly pollute our environment, have consequences for human health about which there is continuing ignorance and debate. Whereas there is considerable ongoing concern about their harmful effects, magnetic fields are at the same time being applied as therapeutic tools in regenerative medicine, oncology, orthopedics, and neurology. This paradox cannot be resolved until the cellular mechanisms underlying such effects are identified. Here, we show by biochemical and imaging experiments that exposure of mammalian cells to weak pulsed electromagnetic fields (PEMFs) stimulates rapid accumulation of reactive oxygen species (ROS), a potentially toxic metabolite with multiple roles in stress response and cellular ageing. Following exposure to PEMF, cell growth is slowed, and ROS-responsive genes are induced. These effects require the presence of cryptochrome, a putative magnetosensor that synthesizes ROS. We conclude that modulation of intracellular ROS via cryptochromes represents a general response to weak EMFs, which can account for either therapeutic or pathological effects depending on exposure. Clinically, our findings provide a rationale to optimize low field magnetic stimulation for novel therapeutic applications while warning against the possibility of harmful synergistic effects with environmental agents that further increase intracellular ROS.
https://www.researchgate.net/publication/328036870_Low-intensity_electromagnetic_fields_induce_human_cryptochrome_to_modulate_intracellular_reactive_oxygen_species
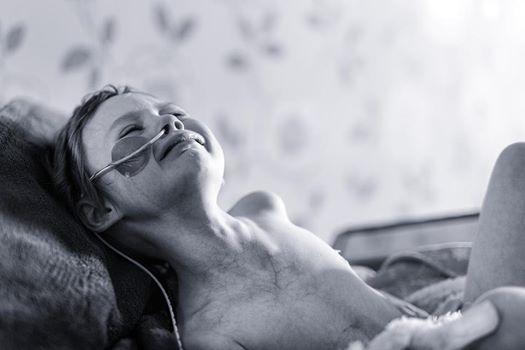
An attempt to wake up the medical community to accept research done in the last 100 years proving that electromagnetic energy can replace brutal chemotherapy. Photo taken by a professional photographer, of his own daughter being treated for Neuroblastoma. The power of the image encouraged Andy to share it with others in order to highlight the 'real' face of childhood cancer. She died. The average cost for such treatment is in the order of 500k+.
Friday, November 30, 2018
Wednesday, November 28, 2018
Oncology and Biophysics: A Need for Integration
Excerpt: "Although we have gone to great lengths to investigate the role of biochemical signaling pathways in tumor development and progression, we have ignored the role of endogenous bioelectric signals that emanate from cell membrane ion channels, pumps and gap junctions. These endogenous signals create electromagnetic fields that have long range effects on cell behavior and gene expression [23]. Failure to include them in the study of cancer development and progression leaves a large knowledge gap that is hindering progress in clinical therapeutics"
Oncology and Biophysics: A Need for Integration
https://www.researchgate.net/publication/263532996_Oncology_and_Biophysics_A_Need_for_Integration
Oncology and Biophysics: A Need for Integration
https://www.researchgate.net/publication/263532996_Oncology_and_Biophysics_A_Need_for_Integration
Saturday, November 24, 2018
Specific electromagnetic frequencies: expression of anti-inflammatory mediators and in cell differentiation
In this study, we investigated the effects of specific low frequency electromagnetic fields sequences on U937 cells, an in vitro model of human monocyte/macrophage differentiation.
U937 cells were exposed to electromagnetic stimulation by means of the SynthéXer® system using two similar sequences, XR-BC31 and XR-BC31/F.
Each sequence was a time series of twenty-nine wave segments.
Here, we report that exposure (4 days, once a day) of U937 cells to the XR-BC31 setting, but not to the XR-BC31/F, resulted in increased expression of the histone demethylase KDM6B along with a global reduction in histone H3 lysine 27 (H3K27) tri-methylation.
Furthermore, exposure to the XR-BC31 sequence induced differentiation of U937 cells towards a macrophage-like phenotype displaying a KDM6B dependent increase inexpression and secretion of the anti-inflammatory interleukins (ILs), IL-10 and IL-4.
Importantly, all the observed changes were highly dependent on the sequence’s nature.
Our results open a new way of interpretation for the effects of low frequency electromagnetic fields observed in vivo.
Indeed, it is conceivable that a specific low frequency electro-magnetic fields treatment may cause changes in chromatin accessibility and consequently in the expression of anti-inflammatory mediators and in cell differentiation.
Wednesday, November 21, 2018
Magnets with Proton Therapy: Analytical investigation of magnetic field effects on Proton lateral deflection and penetrating depth in the water phantom: A relativistic approach
Background
Integrated proton therapy - MRI systems are capable of delivering high doses to the target tissues near sensitive organs and achieve better therapeutic results; however, the applied magnetic field for imaging, influences the protons path, changes the penetration depth and deflects the particles, laterally, leading to dose distribution variations.
Objective
To determine the effects of a magnetic field on the range and the lateral deflection of protons, analytically.
Methods
An analytical survey based on protons energy and range power law relation, without using small angle assumption was done. The penetration depth and lateral deflection of protons with therapeutic energy ranges 60–250 MeV in the presence of uniform magnetic fields of 0–10T intensities, were calculated analytically. Calculations were done for relativistic conditions with Mathematica software version 7.0, and MATLAB 7.0 was applied to plot curves and curve fittings.
Results
In the presence of a magnetic field, the depth of Bragg peak was decreased and it was shifted laterally. A second order polynomial model with power equation for its coefficients and a power model with quadratic polynomial coefficients predicted the maximum lateral deflection (ymax) and maximum penetration depth (zmax) variations with energy and magnetic field intensity, respectively.
Conclusion
The applied correction for deflection angle will give more reliable results in initial energy of 250 MeV and 3T magnetic field intensity. For lower energies and magnetic field intensities the differences are negligible, clinically.
Sunday, November 18, 2018
Flowing cells through pulsed electric fields efficiently purges stem cell preparations of contaminating myeloma cells while preserving stem cell function
Autologous stem cell transplantation, in
the setting of hematologic malignancies
such as lymphoma, improves disease free
survival if the graft has undergone
tumor purging.
Here we show that flowing hematopoietic cells through pulsed electric fields (PEFs) effectively purges myeloma cells without sacrificing functional stem cells.
Electric fields can induce irreversible cell membrane pores in direct relation to cell diameter, an effect we exploit in a flowing system appropriate for clinical scale.
Multiple myeloma (MM) cell lines admixed with human bone marrow (BM) or peripheral blood (PB) cells were passed through PEFs at 1.35 kV/cm to 1.4 kV/cm, resulting in 3- to 4-log tumor cell depletion by flow cytometry and 4.5- to 6-log depletion by tumor regrowth cultures.
Samples from patients with MM gave similar results by cytometry. Stem cell engraftment into nonobese diabetic– severe combined immunodeficient (NOD/ SCID)/ 2m/ mice was unperturbed by PEFs.
Flowing cells through PEFs is a promising technology for rapid tumor cell purging of clinical progenitor cell preparations. (Blood. 2005;105:2235-2238)
http://www.bloodjournal.org/content/bloodjournal/105/5/2235.full.pdf?sso-checked=true
Here we show that flowing hematopoietic cells through pulsed electric fields (PEFs) effectively purges myeloma cells without sacrificing functional stem cells.
Electric fields can induce irreversible cell membrane pores in direct relation to cell diameter, an effect we exploit in a flowing system appropriate for clinical scale.
Multiple myeloma (MM) cell lines admixed with human bone marrow (BM) or peripheral blood (PB) cells were passed through PEFs at 1.35 kV/cm to 1.4 kV/cm, resulting in 3- to 4-log tumor cell depletion by flow cytometry and 4.5- to 6-log depletion by tumor regrowth cultures.
Samples from patients with MM gave similar results by cytometry. Stem cell engraftment into nonobese diabetic– severe combined immunodeficient (NOD/ SCID)/ 2m/ mice was unperturbed by PEFs.
Flowing cells through PEFs is a promising technology for rapid tumor cell purging of clinical progenitor cell preparations. (Blood. 2005;105:2235-2238)
http://www.bloodjournal.org/content/bloodjournal/105/5/2235.full.pdf?sso-checked=true
Saturday, November 17, 2018
Short Exposures to an Extremely Low-Frequency Magnetic Field (ELF MF) Enhance Protein but not mRNA Alkaline Phosphatase Expression in Human Osteosarcoma Cells
Abstract
Background:
Among electromagnetic fields treatments used in orthopedics, extremely low-frequency magnetic fields (ELF MF) need more detailed information about the molecular mechanisms of their effects and exposure conditions.
Objective:
Evaluation of the effects of an ELF MF exposure system, recently introduced among current clinical treatments for fracture healing and other bone diseases, on Alkaline Phosphatase (ALP) activity and expression in a human osteosarcoma cell line (SaOS-2), as marker typically associated to osteogenesis and bone tissue regeneration.
Method:
Cells were exposed to the ELF MF physical stimulus (75 Hz, 1.5 mT) for 1h. Cell viability, enzymatic activity, protein and mRNA expression of alkaline phosphatase were then measured at different times after exposure (0, 4 and 24 h).
Results:
Data demonstrate that this signal is active on an osteogenic process already one hour after exposure. Treatment was, in fact, capable, even after an exposure shorter than those commonly used in clinical applications, to significantly up-regulate alkaline phosphatase enzymatic activity. This regulation is produced essentially through an increase of ALP protein level, without changes of its mRNA concentration, while assessed magnetic field did not affect cell growth and viability and did not produce temperature variations.
Conclusion:
Tested low-frequency magnetic field affects cellular ALP expression with a posttranslational mechanism, without the involvement of regulations at gene transcription and mRNA level. This molecular effect is likely produced even within treated tissues during therapies with this signal and may be implicated in the induction of observed effects in treated patients.https://benthamopen.com/FULLTEXT/TOBIOCJ-12-65
Friday, November 16, 2018
Biological effects of non-ionizing electromagnetic fields: Two sides of a coin
"For example, there are lots of publications indicating that EMF can induce apoptosis and DNA strand-breaks in cells.
On the other hand, these effects could rather be beneficial, in that they could be effectively harnessed for treatment of various disorders, including cancer.
This review discusses and analyzes the results of various in vitro, in vivo and epidemiological studies on the effects of non-ionizing EMFs on cells and organs, including the consequences of exposure to the low and high frequencies EM spectrum.
Emphasis is laid on the analysis of recent data on the role of EMF in the induction of oxidative stress and DNA damage.
Additionally, the impact of EMF on the reproductive system has been discussed, as well as the relationship between EM radiation and blood cancer.
Apart from adverse effects, the therapeutic potential of EMFs for clinical use in different pathologies is also highlighted."
Quantum Magnetic Resonance Therapy: Targeting Biophysical Cancer Vulnerabilities to Effectively Treat and Palliate
Background: Radical paradigm shifts in traditional thinking is paramount to winning the war on cancer and understanding why this disease survives even the most brutal of toxic therapies. There is mounting evidence that biophysical signals are integral to the cycle of initiation, progression and death of cancer cells. Innovative technologies that manipulate this vulnerability in solid tumors could effectively be used to perturb only diseased cells and tissues. Not compromising normally functioning cells while controlling tumor progression, is the ultimate goal for evolving cancer therapeutics like Quantum Magnetic Resonance Therapy, headed promisingly in that direction.
Methods: A patented, CE marked device, the CYTOTRON® delivers rotating, target-specific, modulated, safe Radio Frequencies in the presence of an integrated, instantaneous magnetic field. The presumed modulation of the transmembrane potential of tumor cells and downstream cellular signalling by RF for tissue degeneration in cancer underlies Rotational Field Quantum Magnetic Resonance platform technology. Whole body MRI for tissue proton density determinations was used to compute individualized dosimetry to target solitary or multiple regions of interest in the whole body, simultaneously. Exposure to QMRT was for 1 hour daily for 28 consecutive days. Quality of Life assessments, overall survival and tumor stability using RECIST v1.1 were followed up for 12 months.
Results: Significant increase in life expectancy from the predicted to the actual mean (p=2.13 E-12), and improvements in Karnofsky Performance Scale scores (p=7.25 E-06) and Quality of Life scores (p=1.71 E-08 and p=1.91 E-06) were noted. Thirty six of 51 (71 %) terminally ill patients had stable disease one month after completion of QMRT or longer.
Conclusions: Exposure to radiofrequency-mediated QMRT improved life expectancy and quality of life, along with arrest of tumor progression. This therapy can be safely positioned in a palliative care setting, transitioning to mainstream cancer care with more rigorous clinical validation.
Tuesday, November 13, 2018
Favourable and Unfavourable EMF Frequency Patterns in Cancer: Perspectives for Improved Therapy and Prevention
Carcinogenesis fits in a frequency pattern of electromagnetic field (EMF) waves, in which a gradual loss of cellular organization occurs.
Such generation of cancer features can be inhibited by adequate exposure to coherent electromagnetic frequencies.
However, cancer can also be initiated and promoted at other distinct frequencies of electromagnetic waves.
Both observations were revealed by analyzing 100 different EMF frequency data reported in a meta-analyses of 123 different, earlier published, biomedical studies.
The studied EM frequencies showed a fractal pattern of 12 beneficial (anti-cancer) frequencies, and 12 detrimental (cancer promoting) frequencies, that form the central pattern of a much wider self-similar EMF spectrum of cancer inhibiting or promoting activities.
Inhibiting of the cancer process, and even curing of the disease, can thus be considered through exposure to the coherent type of EM fields.
Stabilization of the disease can be understood by constructive resonance of macromolecules in the cancer cell with the externally appied coherent EMF field frequencies, called solitons/polarons.
The latter, for instance, have been shown earlier to induce repair in DNA/RNA conformation and/or epigenetic changes.
The field of EMF treatment of cancer disorders is rapidly expanding and our studies may invite further experimental and clinical studies in which systematically various potential EMF treatment protocols could be applied, with combined and modulated frequencies, to obtain even more efficient EMF anti-cancer therapies.
https://www.scirp.org/journal/PaperInformation.aspx?paperID=82944
Such generation of cancer features can be inhibited by adequate exposure to coherent electromagnetic frequencies.
However, cancer can also be initiated and promoted at other distinct frequencies of electromagnetic waves.
Both observations were revealed by analyzing 100 different EMF frequency data reported in a meta-analyses of 123 different, earlier published, biomedical studies.
The studied EM frequencies showed a fractal pattern of 12 beneficial (anti-cancer) frequencies, and 12 detrimental (cancer promoting) frequencies, that form the central pattern of a much wider self-similar EMF spectrum of cancer inhibiting or promoting activities.
Inhibiting of the cancer process, and even curing of the disease, can thus be considered through exposure to the coherent type of EM fields.
Stabilization of the disease can be understood by constructive resonance of macromolecules in the cancer cell with the externally appied coherent EMF field frequencies, called solitons/polarons.
The latter, for instance, have been shown earlier to induce repair in DNA/RNA conformation and/or epigenetic changes.
The field of EMF treatment of cancer disorders is rapidly expanding and our studies may invite further experimental and clinical studies in which systematically various potential EMF treatment protocols could be applied, with combined and modulated frequencies, to obtain even more efficient EMF anti-cancer therapies.
https://www.scirp.org/journal/PaperInformation.aspx?paperID=82944
Exposure to a specific time‐varying electromagnetic field inhibits cell proliferation via cAMP and ERK signaling in cancer cells
Exposure to specific electromagnetic field (EMF) patterns can affect a variety of biological systems. We have shown that exposure to Thomas‐EMF, a low‐intensity, frequency‐modulated (25–6 Hz) EMF pattern, inhibited growth and altered cell signaling in malignant cells. Exposure to Thomas‐EMF for 1 h/day inhibited the growth of malignant cells including B16‐BL6 mouse melanoma cells, MDA‐MB‐231, MDA‐MB‐468, BT‐20, and MCF‐7 human breast cancer and HeLa cervical cancer cells but did not affect non‐malignant cells. The Thomas‐EMF‐dependent changes in cell proliferation were mediated by adenosine 3′,5′‐cyclic monophosphate (cAMP) and extracellular‐signal‐regulated kinase (ERK) signaling pathways. Exposure of malignant cells to Thomas‐EMF transiently changed the level of cellular cAMP and promoted ERK phosphorylation. Pharmacologic inhibitors (SQ22536) and activators (forskolin) of cAMP production both blocked the ability of Thomas‐EMF to inhibit cell proliferation, and an inhibitor of the MAP kinase pathway (PD98059) was able to partially block Thomas‐EMF‐dependent inhibition of cell proliferation. Genetic modulation of protein kinase A (PKA) in B16‐BL6 cells also altered the effect of Thomas‐EMF on cell proliferation. Cells transfected with the constitutively active form of PKA (PKA‐CA), which interfered with ERK phosphorylation, also interfered with the Thomas‐EMF effect on cell proliferation. The non‐malignant cells did not show any EMF‐dependent changes in cAMP levels, ERK phosphorylation, or cell growth. These data indicate that exposure to the specific Thomas‐EMF pattern can inhibit the growth of malignant cells in a manner dependent on contributions from the cAMP and MAP kinase pathways.
https://onlinelibrary.wiley.com/doi/abs/10.1002/bem.22096
https://onlinelibrary.wiley.com/doi/abs/10.1002/bem.22096
Sunday, November 11, 2018
Folding of biological proteins
The current geometric and thermodynamic approaches in protein folding studies do not provide a definite solution to understanding mechanisms of folding of biological proteins.
A major problem is that the protein is first synthesized as a linear molecule that subsequently must reach its native configuration in an extremely short time.
Hydrophobicity-hydrophilicity models and random search mechanism cannot explain folding to the 3-D functional form in less than 1 second, as it occurs in the intact cell.
We propose an integral approach, based on the embedding of proteins in the whole cellular context under the postulate: a life protein is never alone.
In this concept the protein molecule is influenced by various long and short distance force fields of nature such as coherent electromagnetic waves and zero-point energy.
In particular, the role of solitons is reviewed in relation to a novel GM-scale biophysical principle, revealed by us.
This recent finding of a set of discrete EM frequency bands, that either promote or endanger life conditions, could be a key in further studies directed at the morphogenetic aspects of protein folding in a biological evolutionary context.
In addition, an alternative hypothesis is presented in which each individual cell may store integral 3-D information holographically at the virtual border of a 4-D hypersphere that surrounds each living cell, providing a field receptive memory structure that is instrumental in guiding the folding process towards coherently oscillating protein networks that are crucial for cell survival.
Saturday, November 10, 2018
Possible repeat, worth it: The antitumor effect of static and extremely low frequency magnetic fields against nephroblastoma and neuroblastoma.
Certain magnetic fields (MF) have potential therapeutic antitumor effect whereas the underlying mechanism remains undefined. In this study, a well-characterized MF was applied to two common childhood malignancies, nephroblastoma and neuroblastoma. This MF has a time-averaged total intensity of 5.1 militesla (mT), and was generated as a superimposition of a static and an extremely low frequency (ELF) MF in 50 Hertz (Hz). In nephroblastoma and neuroblastoma cell lines including G401, CHLA255, and N2a, after MF exposure of 2 h per day, the cell viability decreased significantly after 2 days. After 3 days, inhibition rates of 17-22% were achieved in these cell lines. Furthermore, the inhibition rate was positively associated with exposure time. On the other hand, when using static MF only while maintaining the same time-averaged intensity of 5.1 mT, the inhibition rate was decreased. Thus, both time and combination of ELF field were positively associated with the inhibitory effect of this MF. Exposure to the field decreased cell proliferation and induced apoptosis. Combinational use of MF together with chemotherapeutics cisplatin (DDP) was performed in both in vitro and in vivo experiments. In cell lines, combinational treatment further increased the inhibition rate compared with single use of either DDP or MF. In G401 nephroblastoma tumor model in nude mice, combination of MF and DDP resulted in significant decrease of tumor mass, and the side effect was limited in mild liver injury. MF exposure by itself did not hamper liver or kidney functions. In summary, the antitumor effect of an established MF against neuroblastoma and nephroblastoma is reported, and this field has the potential to be used in combination with DDP to achieve increased efficacy and reduce side effects in these two childhood malignancies.
https://www.ncbi.nlm.nih.gov/pubmed/29719057
https://www.ncbi.nlm.nih.gov/pubmed/29719057
Thursday, November 8, 2018
A review on the use of magnetic fields and ultrasound for non-invasive cancer treatment
Current popular cancer treatment options, include tumor surgery, chemotherapy, and hormonal treatment.
These treatments are often associated with some inherent limitations.
For instances, tumor surgery is not effective in mitigating metastases; the anticancer drugs used for chemotherapy can quickly spread throughout the body and is ineffective in killing metastatic cancer cells.
Therefore, several drug delivery systems (DDS) have been developed to target tumor cells, and release active biomolecule at specific site to eliminate the side effects of anticancer drugs.
However, common challenges of DDS used for cancer treatment, include poor site-specific accumulation, difficulties in entering the tumor microenvironment, poor metastases and treatment efficiency.
In this context, non-invasive cancer treatment approaches, with or without DDS, involving the use of light, heat, magnetic field, electrical field and ultrasound appears to be very attractive.
These approaches can potentially improve treatment efficiency, reduce recovery time, eliminate infections and scar formation.
In this review we focus on the effects of magnetic fields and ultrasound on cancer cells and their application for cancer treatment in the presence of drugs or DDS.
“Biological effects of non-ionizing electromagnetic fields: Two sides of a coin”
Controversial, sensational and often contradictory scientific reports have triggered active debates over the biological effects of electromagnetic fields (EMFs) in literature and mass media the last few decades.
This could lead to confusion and distraction, subsequently hampering the development of a univocal conclusion on the real hazards caused by EMFs on humans.
For example, there are lots of publications indicating that EMF can induce apoptosis and DNA strand-breaks in cells.
On the other hand, these effects could rather be beneficial, in that they could be effectively harnessed for treatment of various disorders, including cancer.
This review discusses and analyzes the results of various in vitro, in vivo and epidemiological studies on the effects of non-ionizing EMFs on cells and organs, including the consequences of exposure to the low and high frequencies EM spectrum.
Emphasis is laid on the analysis of recent data on the role of EMF in the induction of oxidative stress and DNA damage.
Additionally, the impact of EMF on the reproductive system has been discussed, as well as the relationship between EM radiation and blood cancer.
Apart from adverse effects, the therapeutic potential of EMFs for clinical use in different pathologies is also highlighted.
Monday, November 5, 2018
Which eigenfrequencies of non-thermalelectromagnetic waves are life-sustaining and which are, incontrast, detrimental for living cells
Very in-depth paper from Russia and the Netherlands, which explains DNA folding, including a reference to several articles such as:
"Recently, a bio-soliton model was proposed (Geesink and Meijer,2016b), that predicts which eigenfrequencies of non-thermalelectromagnetic waves are life-sustaining and which are, incontrast, detrimental for living cells.
The particular effects are exerted by a range of electromagnetic wave eigen-frequencies of one-tenth of a Hertz till Peta Hertz, that show a pattern of twelve bands, and can be positioned on an acoustic frequency scale. "
Excerpt from the paper's conclusion remark:
"It is shown in the present review that bio-molecular folding and a much wider class of biochemical reactions requires an unrealistically large number of steps to find the correct functional state,irrespective of the various models developed.
The reduction of these issues to some classical problems is considered. On this basis,the known folding-reaction paradox was reformulated and generalized by us. The essence of the generalized paradox, that is, in the proposed broader context, lies in the very fact that complex molecules must reach their native configurations (including their related reactions) during an exponentially large time, which clearly contradicts the observed experimental data on folding time.
In our opinion, perspectives for solving the paradox are associated with an explicit incorporation of topology in the physical models of folding. This condition can be achieved, in our view,through the construction of hybrid models based on the geometrical and physical approaches.
We argue that it is crucial in this respect, to approach the item of protein folding from a holistic standpoint, in which all the inter-acting factors and intrinsic conditions of the intact cell should betaken into account (see Table 3).
These include long-range force fields and local cell biological conditions such as structured water complexes, among many other factors. In particular, there is abundant information in literature on the role of soliton waves(phonon/electron quasi particles) that can be instrumental as guiding elements in the various steps in the folding process"
https://www.researchgate.net/publication/320010451_On_a_generalized_Levinthal%27s_paradox_The_role_of_long-_and_short_range_interactions_in_complex_bio-molecular_reactions_including_protein_and_DNA_folding
"Recently, a bio-soliton model was proposed (Geesink and Meijer,2016b), that predicts which eigenfrequencies of non-thermalelectromagnetic waves are life-sustaining and which are, incontrast, detrimental for living cells.
The particular effects are exerted by a range of electromagnetic wave eigen-frequencies of one-tenth of a Hertz till Peta Hertz, that show a pattern of twelve bands, and can be positioned on an acoustic frequency scale. "
Excerpt from the paper's conclusion remark:
"It is shown in the present review that bio-molecular folding and a much wider class of biochemical reactions requires an unrealistically large number of steps to find the correct functional state,irrespective of the various models developed.
The reduction of these issues to some classical problems is considered. On this basis,the known folding-reaction paradox was reformulated and generalized by us. The essence of the generalized paradox, that is, in the proposed broader context, lies in the very fact that complex molecules must reach their native configurations (including their related reactions) during an exponentially large time, which clearly contradicts the observed experimental data on folding time.
In our opinion, perspectives for solving the paradox are associated with an explicit incorporation of topology in the physical models of folding. This condition can be achieved, in our view,through the construction of hybrid models based on the geometrical and physical approaches.
We argue that it is crucial in this respect, to approach the item of protein folding from a holistic standpoint, in which all the inter-acting factors and intrinsic conditions of the intact cell should betaken into account (see Table 3).
These include long-range force fields and local cell biological conditions such as structured water complexes, among many other factors. In particular, there is abundant information in literature on the role of soliton waves(phonon/electron quasi particles) that can be instrumental as guiding elements in the various steps in the folding process"
https://www.researchgate.net/publication/320010451_On_a_generalized_Levinthal%27s_paradox_The_role_of_long-_and_short_range_interactions_in_complex_bio-molecular_reactions_including_protein_and_DNA_folding
Friday, November 2, 2018
Cancer cell lines response to tumor treating fields: results of a meta-analysis
Background
Tumor Treating Fields (TTFields) therapy, an approved modality for the treatment of glioblastoma, is currently being investigated in many other indications.
These alternating electric fields were shown to exert an inhibitory effect in numerous cancer cell lines with some variability in the response of different cell lines.
The goal of the present study is to compare cell lines characteristics based on their response pattern to TTFields.
Material and Methods
TTFields were applied for 72 hours at their optimal frequency with the same nominal intensity (1.7 V/cm) to forty different human cancerous cell lines.
Cell survival and clonogenicity were determined.
Functional analysis of differentially expressed genes and mutations associated with response to TTFields was performed based on data from the Cancer Cell Line Encyclopedia (CCLE) database.
Sensitivity to TTFields was compared with pharmacological profiling (CCLE).
Results
The inhibitory response to TTFields was found to be distributed around an average of 50% with a cytotoxic effects ranging between 14% and 86% reductions in cell counts, and a clonogenic effect ranging between no effect and 88% reduction in the number of colonies.
In line with TTFields anti-mitotic properties, a correlation between treatment efficacy and cell doubling time was demonstrated.
Yet, few cell lines demonstrated enhanced treatment efficacy despite long doubling time suggesting other factors may also be involved in the response to treatment.
Functional analysis of cell line gene expression and mutation data revealed enriched pathways related to DNA damage repair response such as the BRCA1 repair pathway which validate previous data obtained using different methodologies.
Other pathways which were found to be associated with sensitivity to TTFields include: cell migration, hypoxia signaling, oxidative stress.
Pharmacological profiling based on IC50 values, revealed increased sensitivity to: Lapatinib, PHA-665752 and PLX-4720 within the group of cell lines which were less sensitive to TTFields.
Conclusion
This multi parameter, large scale comparison of cancerous cell line response to TTFields demonstrate the broad effectiveness of TTFields in various cell lines and define the optimal frequency to be applied for each cell line.
The data suggest that beside their anti-mitotic properties, TTFields may have effects on other cellular pathways.
The pharmacological profiling may offer a rational for combining specific agents with TTFields in cells which are less sensitive to the electric fields.
https://www.researchgate.net/publication/327753653_P0417_Cancer_cell_lines_response_to_tumor_treating_fields_results_of_a_meta-analysis
Tumor Treating Fields (TTFields) therapy, an approved modality for the treatment of glioblastoma, is currently being investigated in many other indications.
These alternating electric fields were shown to exert an inhibitory effect in numerous cancer cell lines with some variability in the response of different cell lines.
The goal of the present study is to compare cell lines characteristics based on their response pattern to TTFields.
Material and Methods
TTFields were applied for 72 hours at their optimal frequency with the same nominal intensity (1.7 V/cm) to forty different human cancerous cell lines.
Cell survival and clonogenicity were determined.
Functional analysis of differentially expressed genes and mutations associated with response to TTFields was performed based on data from the Cancer Cell Line Encyclopedia (CCLE) database.
Sensitivity to TTFields was compared with pharmacological profiling (CCLE).
Results
The inhibitory response to TTFields was found to be distributed around an average of 50% with a cytotoxic effects ranging between 14% and 86% reductions in cell counts, and a clonogenic effect ranging between no effect and 88% reduction in the number of colonies.
In line with TTFields anti-mitotic properties, a correlation between treatment efficacy and cell doubling time was demonstrated.
Yet, few cell lines demonstrated enhanced treatment efficacy despite long doubling time suggesting other factors may also be involved in the response to treatment.
Functional analysis of cell line gene expression and mutation data revealed enriched pathways related to DNA damage repair response such as the BRCA1 repair pathway which validate previous data obtained using different methodologies.
Other pathways which were found to be associated with sensitivity to TTFields include: cell migration, hypoxia signaling, oxidative stress.
Pharmacological profiling based on IC50 values, revealed increased sensitivity to: Lapatinib, PHA-665752 and PLX-4720 within the group of cell lines which were less sensitive to TTFields.
Conclusion
This multi parameter, large scale comparison of cancerous cell line response to TTFields demonstrate the broad effectiveness of TTFields in various cell lines and define the optimal frequency to be applied for each cell line.
The data suggest that beside their anti-mitotic properties, TTFields may have effects on other cellular pathways.
The pharmacological profiling may offer a rational for combining specific agents with TTFields in cells which are less sensitive to the electric fields.
https://www.researchgate.net/publication/327753653_P0417_Cancer_cell_lines_response_to_tumor_treating_fields_results_of_a_meta-analysis
Thursday, November 1, 2018
Low frequency pulsed electromagnetic field promotes the recovery of neurological function after spinal cord injury in rats.
Low frequency pulsed electromagnetic field (LFPEMF) has been shown to provide anti-inflammatory and antioxidative effects.
However, there are no reports on whether LFPEMF can treat spinal cord injury (SCI) and its therapeutic mechanism.
Therefore, this study was conducted to investigate whether LFPEMF can promote the recovery of neurological function after SCI in rats and its therapeutic mechanism.
Basso-Beattie-Bresnahan (BBB) score and transcranial magnetic motor-evoked potentials (tcMMEPs) were recorded to assess the recovery of neurological function.
Hematoxylin and eosin (HE) staining and luxol fast blue (LFB) staining were performed to assess the severity of SCI.
Immunofluorescence (IF) staining and western blotting (WB) were performed to assess the differentiation of oligodendrocyte precursor cells (OPCs) into oligodendrocytes (OLs).
Toluidine blue (TB) staining was performed to assess remyelination.
WB and enzyme-linked immunosorbent assays (ELISA) were performed to assess the expression of neurotrophins and inflammatory factors.
Our results showed that following stimulation by LFPEMF, there were significant improvements in BBB scores, tcMMEP amplitudes, the extent of the damage, and reduced demyelination in rats after SCI.
The mature OLs, the number of well-myelinated fibers, and the myelin sheath thickness significantly increased in rats stimulated by LFPEMF after SCI.
The expression of neurotrophins significantly increased, and the expression of inflammatory factors significantly decreased in rats stimulated by LFPEMF after SCI.
Therefore, we suggest that LFPEMF can promote the recovery of neurological function in rats after SCI by improving the differentiation of OPCs into OLs and promoting remyelination, as well as by inhibiting inflammation and promoting neurotrophic effects.
Subscribe to:
Posts (Atom)